AI transcript
0:00:04 Hi, everyone. Welcome to this week’s episode of 16 Minutes, where we cover what’s going
0:00:08 on, what’s in the news from our vantage point in tech. In this episode, we’re going to
0:00:13 go deep on one topic, which is the coronavirus, and it’s a very fast-developing news cycle,
0:00:17 so we’re going to take a snapshot for where we are right now. And since this show is all
0:00:21 about teasing apart what’s hype, what’s real, given all the buzz and headlines out there,
0:00:24 we’re going to try to focus on what we know and what we don’t know. I’ve tried drawing
0:00:30 wherever possible from primary sources, so CDC reports, World Health Organization reports,
0:00:35 etc., instead of only looking at news headlines and derivative reports. And our ASICs and
0:00:38 the expert, who I’ll introduce in a moment, will be bringing in the vantage point coming
0:00:41 from bioengineering and that aspect as well.
0:00:45 So first of all, let me quickly summarize the news. People are referring to this outbreak
0:00:50 as the coronavirus, but it’s actually a new type of coronavirus because coronavirus is
0:00:55 actually the general term for a more common category of viruses. And this current strain
0:01:03 is called 2019-NCOV for 2019-N novel coronavirus. It’s a rapidly developing situation, but
0:01:10 as of January 26, according to the situation update on the World Health Organization website,
0:01:16 there’s a total of 2014 confirmed cases that have been reported globally. Of these, 98%
0:01:24 were reported from China, including Hong Kong, Macau, and Taipei. 324 of 1,975 cases have
0:01:30 been reported as severely ill, with 56 deaths reported to that date. Finally, 29 confirmed
0:01:35 cases have been reported out of China in 10 countries, and in the table that the World
0:01:40 Health Organization provided, there’s two cases listed in the US, but there’s more.
0:01:43 Again, this is from the Six Situations Report, which comes out every few days, and this one
0:01:47 came out on Sunday, January 26. That’s a very high-level summary. Now, let me welcome Judy
0:01:52 Sovitzkaya on the A6nz BioDeal team. First of all, really quickly, what is it? What is
0:01:53 the coronavirus?
0:02:00 Yeah, so let’s discuss what even is a virus. So a virus is basically a bunch of DNA or
0:02:05 RNA, some sort of nucleic acid, surrounded by a protein shell called the capsid of the
0:02:09 virus. That is the entire organism. And a lot of people actually don’t even call this
0:02:13 an organism because it’s not quite alive and it’s not quite dead. It’s something in
0:02:14 between.
0:02:17 There’s kind of a debate in the scientific community and the philosophical community
0:02:22 about what is a living thing. And the place where most people have come down is that you
0:02:27 need two conditions to be alive. You need to metabolize, which means you’re taking some
0:02:31 chemicals, transforming them into other chemicals, and pulling out energy in the process and
0:02:37 using that energy for something. And the second requirement for a living thing is to multiply,
0:02:43 to reproduce. So viruses really only satisfy the second condition. They don’t do anything
0:02:47 on their own. They don’t… Outside of a human host, they are non-living.
0:02:52 So that’s why there’s such a debate. The bottom line is that they don’t metabolize, but they
0:02:53 do multiply.
0:02:54 Exactly.
0:02:58 So tell me now more about the coronavirus category.
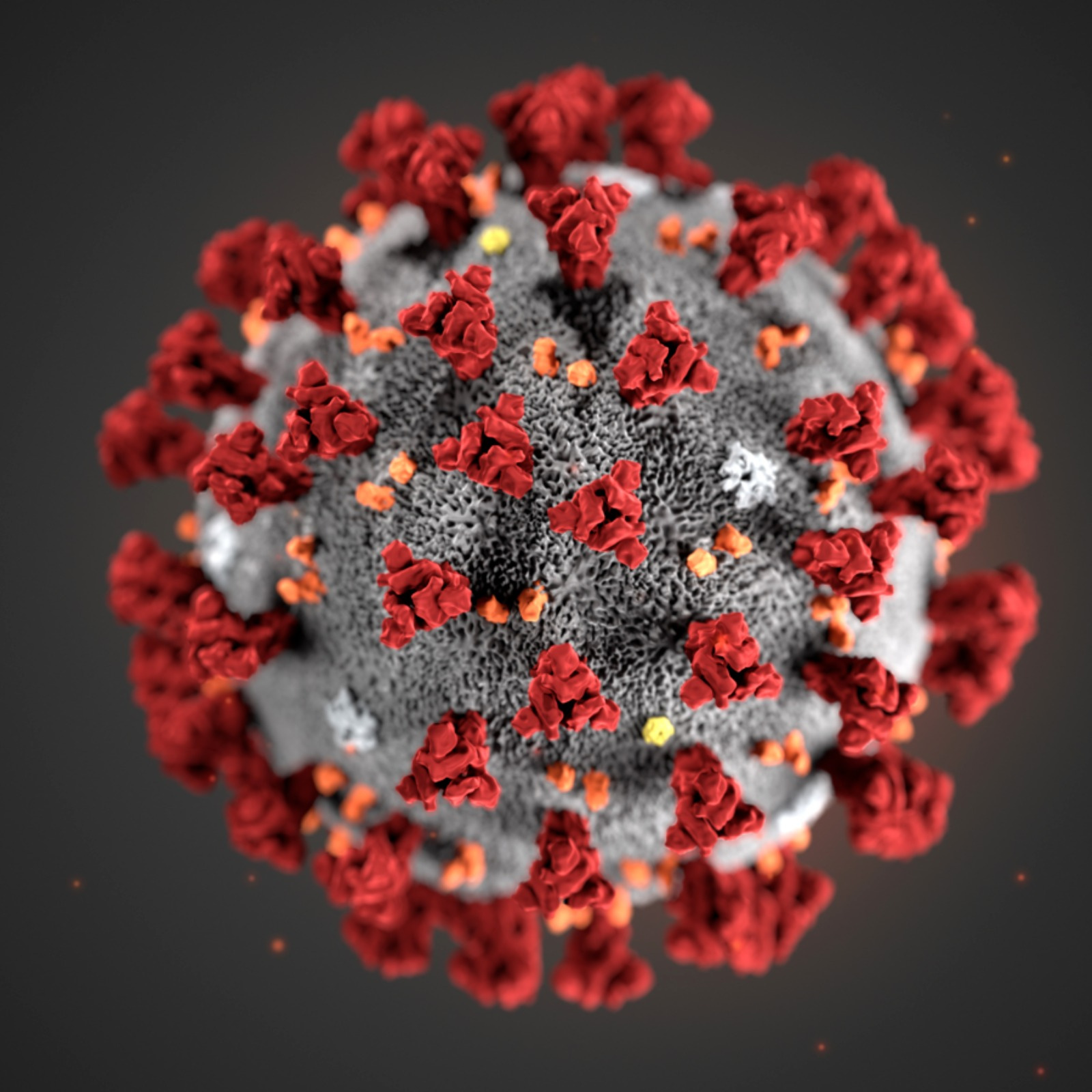
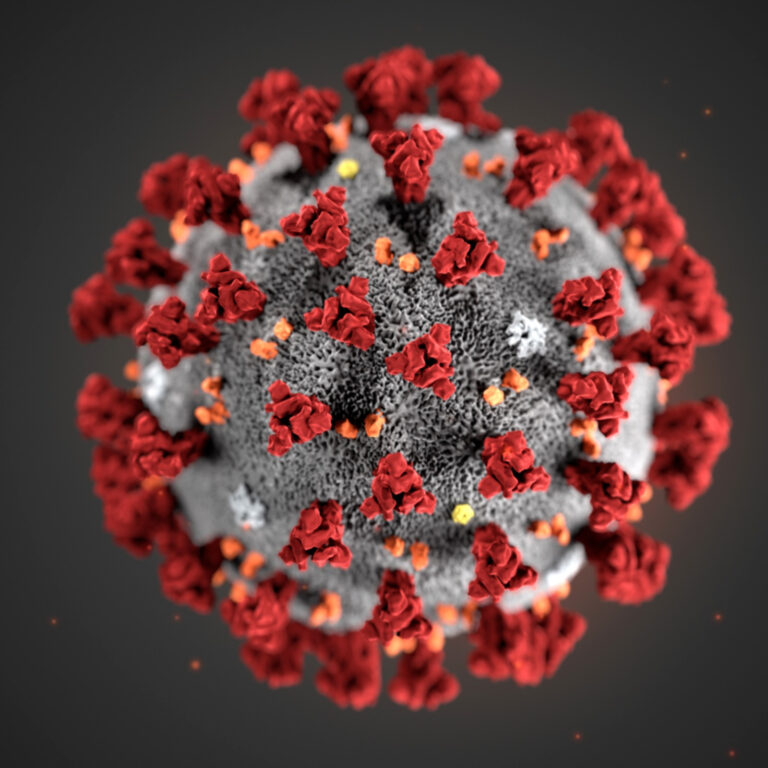
0:03:04 So the reason it’s called coronavirus is because on electron microscopy images, it actually
0:03:09 looks like there’s a little crown around the virus. The capsid for the coronavirus has
0:03:14 these proteins on it that are spikes. And a lot of the ways that we’re developing vaccines
0:03:17 against this virus and a lot of the ways that we’re identifying different types of these
0:03:21 viruses is by characterizing those spike proteins.
0:03:26 And as with most things in biology, we use Greek symbols to denote different versions
0:03:30 of the coronavirus. So there’s the alpha, the beta, the delta, and the gamma, which
0:03:36 are kind of four of the main categories of coronavirus. A lot of the common viruses are
0:03:39 either alpha or beta types, but SARS and MERS, they’re all betas.
0:03:43 Okay. So that’s kind of scientifically what it is. Now let’s practically break down the
0:03:48 symptoms. According to the CDC, the symptoms can include fever, cough, shortness of breath,
0:03:53 or other respiratory symptoms. And they believe that at this time, that symptoms of this virus
0:04:00 may appear in as few as two days or as long as 14 days after exposure. And this is actually
0:04:04 similar to what’s been seen with the previous incubation period of MERS viruses. And I’ll
0:04:08 get to what that is in a minute. But unlike those viruses, this particular one rarely
0:04:13 produces obvious like runny noses or intestinal symptoms necessarily, just according to one
0:04:18 report and that was recently published in Lancet. So I guess the question is that all
0:04:21 of these things are on a continuum. It’s not very discreet. Like this is all symptoms that
0:04:24 can describe frankly, any common cold, right? It’s the WebMD problem.
0:04:28 Right. Basically, you can Google it and find, associate yourself with anything. So the question
0:04:34 I have is, how does this stack up against MERS and SARS? And just really quickly to
0:04:39 summarize, SARS was the acronym for severe acute respiratory syndrome. There’s a big
0:04:44 outbreak of it in the early 2000s. And then MERS is Middle East respiratory syndrome.
0:04:49 And that is new as of 2012. So coronaviruses cause about 10 to 30% of colds, just your
0:04:54 common colds. And those are not nearly as serious as this disease. And some of the differences
0:05:00 between these epidemic causing coronaviruses versus your common cold is just the severity
0:05:04 of the infection, the likelihood that you are to die or to have really serious complications
0:05:10 from the infection. And in all of these cases, SARS, MERS, and this current coronavirus,
0:05:15 it’s because the coronavirus is infecting the lower part of the respiratory tract versus
0:05:17 just staying around your upper respiratory tract area.
0:05:21 Right. So not just your mouth, nose and sinuses, but by going into your lungs. So just like
0:05:26 a quick summary of where the fatality rates are, SARS apparently claimed about 10% of
0:05:32 people and MERS was much worse, claiming 30% of the people it infected. It’s also interesting
0:05:35 because I’ve been reading a lot of papers, but none of them are peer reviewed. In fact,
0:05:40 one of the papers between Friday and today was already updated with V2, but the authors
0:05:44 of the local institutes of virology, Chinese Academy of Sciences, local hospitals and the
0:05:50 provincial CDCs in China within this area supposedly analyze full length genome sequences
0:05:54 from five patients at the early stage of the outbreak. And they found that almost all of
0:05:59 those were identical to each other. So it’s the same virus and B, that about 79.5%. And
0:06:05 again, this is the current paper still being updated, identify to SARS coronavirus.
0:06:08 So that’s actually the most interesting thing from a bioengineering perspective about this
0:06:14 particular epidemic is how incredibly quickly we have sequenced this virus for past epidemics.
0:06:19 It’s taken time for us to really understand the genome and the molecular nature of a given
0:06:23 virus that’s causing an epidemic. In this case, within two weeks, people had already
0:06:29 published draft versions of the genome sequence for this virus. And the science is happening
0:06:34 in the sort of really live way that doesn’t happen very often where people are commenting
0:06:36 literally in the GenBank on the GenBank website.
0:06:40 The CDC uploaded the entire genome of the virus from the first reported case in the
0:06:44 United States to GenBank. And it’s also interesting because in the age of social media, which
0:06:50 cuts both ways virally, it’s also spreading information much faster. Coronavirus was first
0:06:54 detected in Wuhan city in the Hubei province in China, beginning with 44 patients who had
0:07:00 quote, pneumonia of unknown etiology or unknown cause between New Year’s Eve and the first
0:07:05 couple of days of 2020. And then was identified as a new type of virus isolated by Chinese
0:07:10 authorities on January 7. And then on January 11 and 12, the World Health Organization received
0:07:14 detailed information from the National Health Commission in China that the outbreak is associated
0:07:21 with exposures in one seafood market in Wuhan city. And basically it’s showing the spread
0:07:26 of information. Whereas with the SARS crisis, journalist Helen Branswell at Stat News was
0:07:31 commenting because she had covered the SARS crisis in 2003, that one might be tempted
0:07:35 to say SARS start was worse, it spread faster, not sure that’s true. SARS was well underway
0:07:40 for at least 4.5 months before the world knew there was a new virus spreading. This current
0:07:44 coronavirus seems to have been spotted much, much sooner after its emergence. But what’s
0:07:47 really interesting is not just that it’s been spotted sooner, but that the genetic
0:07:53 information we have is moving much faster. So can you talk to me about what that tells
0:07:54 us and why that matters?
0:07:58 So there’s a couple of areas where you get benefits from having all this genetic information
0:08:03 so quickly. The first is just diagnosis. So if tomorrow somebody in San Francisco was
0:08:07 to go into an urgent care clinic and say that they have a cold, we could really quickly
0:08:12 identify whether or not that actually belongs to this epidemic.
0:08:16 Another advantage we have once we have the genome sequence is that in this age of genomic
0:08:20 medicine that we’re entering, where we’re actually creating vaccines that are based
0:08:26 on genome sequences. The third implication is for figuring out treatments and also predicting
0:08:30 some of the features of the epidemic. So we know in this example that this coronavirus
0:08:35 looks really similar to SARS. So we can look back at the SARS epidemic, understand how
0:08:39 quickly it’s spread, in which populations. And from the genomic information, you can
0:08:44 actually see, for example, the spikes on the corona, which are involved in getting into
0:08:49 cells, do those look similar to what the SARS spikes look like? And maybe the treatments
0:08:51 that work in this case as well.
0:08:54 So the high level summary is that having the genomic information, which we didn’t have
0:08:59 then when we do have now, by then I mean the SARS outbreak, which happened about 2002 to
0:09:05 2004, the peak was 2003, is that you can classify things much more easily, figure out where
0:09:10 it belongs, it doesn’t belong, kind of isolate that, that you can develop things faster based
0:09:14 on it, although there isn’t a vaccine yet, and that you can figure out treatment protocols
0:09:17 based on the similarities and differences.
0:09:21 Was there anything else on the connection between mayors and SARS from a genomics perspective?
0:09:26 Yeah. So because the science is happening live, you’re seeing a lot of pretty quick modifications
0:09:31 to what people have already said. So the first paper analyzing the genome of this virus that
0:09:38 I saw at least, described that the spike proteins that are used to enter into lung cells are
0:09:42 different enough between SARS and this new coronavirus, that they thought it wouldn’t
0:09:47 be as bad as SARS. And then within like literally two days, I think I saw a paper that corrected
0:09:51 that and said that actually the protein is quite similar at the protein level.
0:09:54 Are there any bioengineering implications of that? This goes to me that the eternal
0:09:58 question of how DNA expresses itself practically in the complexity of disease.
0:10:02 That’s a really good question. This mirrors everything in genomics where we thought that
0:10:05 once we have the sequence, we’ll know all the answers. And that’s definitely not the
0:10:09 case. We can know from the DNA what the difference is going to be in the protein. That’s one
0:10:13 to one, there’s no guessing there. But once we know what’s different about the protein,
0:10:18 we don’t yet know quite what that means for how it will behave. So we don’t know if that
0:10:23 difference means it’s stronger or it’s weaker or if it will infect different cells or what
0:10:24 that’s going to mean.
0:10:28 So now let’s move on to more of the details of how it spreads and the measurements of
0:10:33 that spread. So first note is that generally the coronavirus has spread through air. They’re
0:10:39 known as zoonotic in that they originate in animals and only sometimes leap to humans.
0:10:43 And there’s been some speculation that this one seemed to originate in bats, but it’s
0:10:48 usually indirect mechanisms. So in mayors, it went from bats to camels before going to
0:10:52 humans. And a couple of papers have commented on the similarity of this new virus to bat
0:10:58 DNA. Like one found that it’s 96% identical. Another journal of medical virology also
0:11:02 observed similar components, but suggested snakes and many other people are skeptical
0:11:06 of that. The long story short is no one really knows, despite having some genomic information
0:11:07 around it.
0:11:11 But it’s interesting to note that important epidemics come from animal sources and some
0:11:15 speculation about the reason for that is that the viruses have time to evolve in their animal
0:11:19 hosts and they’re evolving away from what human hosts have seen before and what their
0:11:21 immune system has been able to recognize before.
0:11:27 So then let’s talk about the spread. A lot of the articles are talking about R0 or R0.
0:11:28 What does it measure?
0:11:33 So R0 is essentially the number of people that you would expect to get infected from
0:11:34 any single case of infection.
0:11:37 So if the R0 is say five, what does that mean?
0:11:41 That means that you should expect that on average, five people will get sick from one
0:11:45 single person that they come into contact with. So for every person that has the disease,
0:11:47 five additional people get the disease.
0:11:52 And interestingly, these are reported as ranges. So like measles has an R0 of 12 to 18.
0:11:57 Yeah. So measles is super infectious. It’s known as sort of the highest or one of the
0:12:01 highest infectiousness variables, which is why it was so important to have vaccines and
0:12:03 herd immunity for measles specifically.
0:12:08 And the reason that it’s reported as ranges is because they depend on the particular population
0:12:10 and the particular moment in time.
0:12:15 So then let’s just talk about some of the facts of the spread. So the 29 exported cases reported
0:12:21 by the World Health Organization, 26 had a travel history from Wuhan city in China.
0:12:24 And then for two of the three cases that were identified in countries outside of China,
0:12:29 one in Australia had direct contact with the confirmed case from Wuhan while in China.
0:12:33 And one in Vietnam had no travel history, but was in contact with the confirmed case.
0:12:35 His father had a travel history to Wuhan.
0:12:39 So what we do know is that human to human transmission is occurring. The preliminary
0:12:42 are not estimate that was presented at the International Health Regulations Emergency
0:12:49 Committee was a range of 1.4 to 2.5, which relatively to the measles example is not as
0:12:54 crazy bad. SARS had an R not between two to five. So that kind of puts that those numbers
0:12:55 in perspective.
0:12:59 It might be a little premature to set these numbers just because the number of cases is
0:13:04 still been relatively low and they haven’t had time to play themselves out. So we don’t
0:13:08 know if there’s many, many cases out there who have not presented symptoms and therefore
0:13:12 we don’t have clear stats on those people.
0:13:17 The big question here is how bad is this epidemic? That’s the question with every epidemic. And
0:13:20 it’s actually so much more complicated than that because there’s a number of different
0:13:24 variables that go into determining how bad something is going to be. It’s very tempting
0:13:29 to put sort of a single number on how bad on a scale of one to 10, for example, but it
0:13:33 doesn’t really take into account all of the nuance in each particular epidemic.
0:13:38 So our not is calculated from the data is actually an aggregate measure. There’s a lot
0:13:41 of different variables that go into our not. So to break them all down into their individual
0:13:44 components and then we can build them back up into our not.
0:13:48 There’s a lot of variables that are going to matter here. One is how well does the virus
0:13:53 transmit itself? So if it’s airborne, it’s able to multiply or transmit itself substantially
0:13:54 more easily.
0:13:59 Right. Versus like exchanging bodily fluids and which requires a lot of specific contact.
0:14:03 Exactly. Another piece is how is it actually getting into the cells? Is it good at infecting
0:14:09 cells? Is it good at its job essentially? Another question is what is the population that it’s
0:14:15 occurring inside of? Is that population moving a lot? Are people coming into very close contact
0:14:20 with each other? So that’s also going to factor in particularly interesting feature of this
0:14:24 is that it happened during Chinese New Year, which is a period of time when a lot of people
0:14:26 in China travel.
0:14:29 So what I think is really interesting to this and having worked at the Gates Foundation and
0:14:35 seen how we think about epidemics on a global scale is that there’s different, there’s two
0:14:39 orthogonal ways to think about an epidemic, which is how much does it spread and then
0:14:41 how bad is it once you get it.
0:14:44 And you’re saying it’s orthogonal or contradictory or why?
0:14:49 So there’s a notion of case fatality, which is for each infection with what likelihood
0:14:53 will the person die from that infection or have like very serious complications. And that’s
0:14:57 actually completely orthogonal to all of the other variables that we talked about before.
0:15:03 One is that if the virus is actually not that deadly or if it expresses itself in a person
0:15:07 after a substantial incubation sign, it might end up creating a bigger epidemic because
0:15:13 that additional time will allow the patient to infect additional individuals. The are
0:15:19 not for that particular virus might be substantially higher, even though its fatality rate is lower.
0:15:23 So it’s an interesting paradox that you could actually have a virus that is less bad once
0:15:26 you get it, but is more bad on the population scale.
0:15:30 So you have to define the metric by which you’re saying whether or not an epidemic is
0:15:34 bad. Is it the number of people who die? Is it the number of people who are infected?
0:15:38 Is it the extent of the spread geographically? There’s a lot of different ways to think about
0:15:40 how bad an epidemic is.
0:15:44 Now let’s just talk about treatments and concrete things that are happening right now.
0:15:48 So just to quickly summarize what’s happening. The CDC is conducting entry skinnings at five
0:15:55 major airports Atlanta, Chicago, Los Angeles, LAX, New York City, JFK and San Francisco.
0:15:58 Doctors are treating symptoms. There’s no vaccine yet. The CDC has developed a real-time
0:16:06 reverse transcription polymerase chain reaction or RRT-PCR test that can diagnose this virus
0:16:08 in respiratory and serum samples.
0:16:15 So what that is is basically a way of seeing if the nucleic acids, if the patient sample
0:16:19 contains the same sequence as the sequence that we know to be involved with the virus
0:16:23 that we know as a part of the virus. So you don’t have to sequence every single patient.
0:16:28 Okay, and then on January 24th, just a few days ago, the CDC publicly posted the assay
0:16:32 protocol for this test and the quote they said, “Currently testing for this virus must
0:16:36 take place at the CDC, but in the coming days and weeks, they will share these tests with
0:16:40 domestic and international partners. And they’re also growing the virus and cell culture, which
0:16:44 is necessary for further studies, including for additional characterization.”
0:16:49 So concretely now from a practical technology point of view, because obviously this is the
0:16:53 serious crisis. There’s a lot to be done and a lot of different players. Where do you sort
0:16:56 of seeing some of the things that might happen that where tech can help?
0:17:01 What’s really interesting about this moment is that because we have this increase in genomic
0:17:08 medicine, just this past weekend, the Coalition for Epidemic Preparedness Innovations, CEPI,
0:17:13 gave out grants to three different pharmaceutical companies of a total of $12.5 million, and
0:17:17 they’re currently engaged in a race. These companies are targeting dates for releasing
0:17:22 their vaccine of between four to 16 weeks from now, which is just totally unheard of.
0:17:27 For a company to spin up a vaccine for the SARS epidemic in 2003 would have taken months
0:17:32 to years for them to develop the new drug and actually get it approved. In this case,
0:17:36 two of these companies made these types of vaccines for other sequences. They’re able
0:17:40 to take what they’ve already built in-house for their other programs, and they can just
0:17:44 very quickly adapt it to this particular virus. All they have to do is create a drop-in replacement
0:17:49 of the sequence that they’ve already worked on with the sequence of this new coronavirus.
0:17:55 Is it fair to say that it’s not dissimilar to engineering in terms of semiconductor and
0:17:58 manufacturing lines? When you say drop-in sequence, does that mean that it’s basically
0:18:03 a matter of using existing scaling methods and you’re just changing the actual code,
0:18:04 quite literally the biological code?
0:18:09 I think that’s actually a really good way of describing it. It’s changing the code,
0:18:12 but you already have the manufacturing line set up. You already know exactly how you’re
0:18:17 going to make this thing. There may be differences, but they will be minimal compared to the differences
0:18:20 that would have existed with a completely different medicine.
0:18:22 Having to bespoke or custom-make it yourself.
0:18:23 Yeah, exactly.
0:18:28 Bottom-line it for me, Judy. How should we think about this news about the coronavirus
0:18:35 still developing? Just to be very clear. Specifically, this is for NCOV 2019. We’ve
0:18:39 covered the high level of what we know and what we don’t know. What would your bottom-line
0:18:42 be from the perspective of a bioengineer?
0:18:46 The bottom-line is that we really need to think about how we are interacting with people,
0:18:51 how we’re traveling, and how we are protecting ourselves from the virus. From my perspective
0:18:57 as a bioengineer, we’ve been talking about how sequencing and synthesis of DNA is becoming
0:19:02 faster and cheaper every single day. This is an example of that in action. This is not
0:19:06 something that would have been possible even a couple of years ago. I think that what we’re
0:19:10 seeing is the beginning of how quickly and how efficiently we’re going to be able to
0:19:16 get to vaccines in the future as we continue to decrease the cost of sequencing and synthesis.
0:19:20 This is a really interesting time because we’re able to figure out the diagnostics piece,
0:19:25 the vaccine piece, and the treatment piece. It’s still in progress, but we have a huge
0:19:26 head start.
0:19:33 Thank you, Judy. For those of you who would like more information, please visit www.cdc.gov/coronavirus.
0:19:38 I’ve also included the link sources for this episode in the show notes, which you can find
0:19:44 at a6nc.com/16minutes. As a reminder, if you haven’t already subscribed to this separate
0:19:48 show in your podcast feed, please do so now and thank you for listening.
0:00:08 on, what’s in the news from our vantage point in tech. In this episode, we’re going to
0:00:13 go deep on one topic, which is the coronavirus, and it’s a very fast-developing news cycle,
0:00:17 so we’re going to take a snapshot for where we are right now. And since this show is all
0:00:21 about teasing apart what’s hype, what’s real, given all the buzz and headlines out there,
0:00:24 we’re going to try to focus on what we know and what we don’t know. I’ve tried drawing
0:00:30 wherever possible from primary sources, so CDC reports, World Health Organization reports,
0:00:35 etc., instead of only looking at news headlines and derivative reports. And our ASICs and
0:00:38 the expert, who I’ll introduce in a moment, will be bringing in the vantage point coming
0:00:41 from bioengineering and that aspect as well.
0:00:45 So first of all, let me quickly summarize the news. People are referring to this outbreak
0:00:50 as the coronavirus, but it’s actually a new type of coronavirus because coronavirus is
0:00:55 actually the general term for a more common category of viruses. And this current strain
0:01:03 is called 2019-NCOV for 2019-N novel coronavirus. It’s a rapidly developing situation, but
0:01:10 as of January 26, according to the situation update on the World Health Organization website,
0:01:16 there’s a total of 2014 confirmed cases that have been reported globally. Of these, 98%
0:01:24 were reported from China, including Hong Kong, Macau, and Taipei. 324 of 1,975 cases have
0:01:30 been reported as severely ill, with 56 deaths reported to that date. Finally, 29 confirmed
0:01:35 cases have been reported out of China in 10 countries, and in the table that the World
0:01:40 Health Organization provided, there’s two cases listed in the US, but there’s more.
0:01:43 Again, this is from the Six Situations Report, which comes out every few days, and this one
0:01:47 came out on Sunday, January 26. That’s a very high-level summary. Now, let me welcome Judy
0:01:52 Sovitzkaya on the A6nz BioDeal team. First of all, really quickly, what is it? What is
0:01:53 the coronavirus?
0:02:00 Yeah, so let’s discuss what even is a virus. So a virus is basically a bunch of DNA or
0:02:05 RNA, some sort of nucleic acid, surrounded by a protein shell called the capsid of the
0:02:09 virus. That is the entire organism. And a lot of people actually don’t even call this
0:02:13 an organism because it’s not quite alive and it’s not quite dead. It’s something in
0:02:14 between.
0:02:17 There’s kind of a debate in the scientific community and the philosophical community
0:02:22 about what is a living thing. And the place where most people have come down is that you
0:02:27 need two conditions to be alive. You need to metabolize, which means you’re taking some
0:02:31 chemicals, transforming them into other chemicals, and pulling out energy in the process and
0:02:37 using that energy for something. And the second requirement for a living thing is to multiply,
0:02:43 to reproduce. So viruses really only satisfy the second condition. They don’t do anything
0:02:47 on their own. They don’t… Outside of a human host, they are non-living.
0:02:52 So that’s why there’s such a debate. The bottom line is that they don’t metabolize, but they
0:02:53 do multiply.
0:02:54 Exactly.
0:02:58 So tell me now more about the coronavirus category.
0:03:04 So the reason it’s called coronavirus is because on electron microscopy images, it actually
0:03:09 looks like there’s a little crown around the virus. The capsid for the coronavirus has
0:03:14 these proteins on it that are spikes. And a lot of the ways that we’re developing vaccines
0:03:17 against this virus and a lot of the ways that we’re identifying different types of these
0:03:21 viruses is by characterizing those spike proteins.
0:03:26 And as with most things in biology, we use Greek symbols to denote different versions
0:03:30 of the coronavirus. So there’s the alpha, the beta, the delta, and the gamma, which
0:03:36 are kind of four of the main categories of coronavirus. A lot of the common viruses are
0:03:39 either alpha or beta types, but SARS and MERS, they’re all betas.
0:03:43 Okay. So that’s kind of scientifically what it is. Now let’s practically break down the
0:03:48 symptoms. According to the CDC, the symptoms can include fever, cough, shortness of breath,
0:03:53 or other respiratory symptoms. And they believe that at this time, that symptoms of this virus
0:04:00 may appear in as few as two days or as long as 14 days after exposure. And this is actually
0:04:04 similar to what’s been seen with the previous incubation period of MERS viruses. And I’ll
0:04:08 get to what that is in a minute. But unlike those viruses, this particular one rarely
0:04:13 produces obvious like runny noses or intestinal symptoms necessarily, just according to one
0:04:18 report and that was recently published in Lancet. So I guess the question is that all
0:04:21 of these things are on a continuum. It’s not very discreet. Like this is all symptoms that
0:04:24 can describe frankly, any common cold, right? It’s the WebMD problem.
0:04:28 Right. Basically, you can Google it and find, associate yourself with anything. So the question
0:04:34 I have is, how does this stack up against MERS and SARS? And just really quickly to
0:04:39 summarize, SARS was the acronym for severe acute respiratory syndrome. There’s a big
0:04:44 outbreak of it in the early 2000s. And then MERS is Middle East respiratory syndrome.
0:04:49 And that is new as of 2012. So coronaviruses cause about 10 to 30% of colds, just your
0:04:54 common colds. And those are not nearly as serious as this disease. And some of the differences
0:05:00 between these epidemic causing coronaviruses versus your common cold is just the severity
0:05:04 of the infection, the likelihood that you are to die or to have really serious complications
0:05:10 from the infection. And in all of these cases, SARS, MERS, and this current coronavirus,
0:05:15 it’s because the coronavirus is infecting the lower part of the respiratory tract versus
0:05:17 just staying around your upper respiratory tract area.
0:05:21 Right. So not just your mouth, nose and sinuses, but by going into your lungs. So just like
0:05:26 a quick summary of where the fatality rates are, SARS apparently claimed about 10% of
0:05:32 people and MERS was much worse, claiming 30% of the people it infected. It’s also interesting
0:05:35 because I’ve been reading a lot of papers, but none of them are peer reviewed. In fact,
0:05:40 one of the papers between Friday and today was already updated with V2, but the authors
0:05:44 of the local institutes of virology, Chinese Academy of Sciences, local hospitals and the
0:05:50 provincial CDCs in China within this area supposedly analyze full length genome sequences
0:05:54 from five patients at the early stage of the outbreak. And they found that almost all of
0:05:59 those were identical to each other. So it’s the same virus and B, that about 79.5%. And
0:06:05 again, this is the current paper still being updated, identify to SARS coronavirus.
0:06:08 So that’s actually the most interesting thing from a bioengineering perspective about this
0:06:14 particular epidemic is how incredibly quickly we have sequenced this virus for past epidemics.
0:06:19 It’s taken time for us to really understand the genome and the molecular nature of a given
0:06:23 virus that’s causing an epidemic. In this case, within two weeks, people had already
0:06:29 published draft versions of the genome sequence for this virus. And the science is happening
0:06:34 in the sort of really live way that doesn’t happen very often where people are commenting
0:06:36 literally in the GenBank on the GenBank website.
0:06:40 The CDC uploaded the entire genome of the virus from the first reported case in the
0:06:44 United States to GenBank. And it’s also interesting because in the age of social media, which
0:06:50 cuts both ways virally, it’s also spreading information much faster. Coronavirus was first
0:06:54 detected in Wuhan city in the Hubei province in China, beginning with 44 patients who had
0:07:00 quote, pneumonia of unknown etiology or unknown cause between New Year’s Eve and the first
0:07:05 couple of days of 2020. And then was identified as a new type of virus isolated by Chinese
0:07:10 authorities on January 7. And then on January 11 and 12, the World Health Organization received
0:07:14 detailed information from the National Health Commission in China that the outbreak is associated
0:07:21 with exposures in one seafood market in Wuhan city. And basically it’s showing the spread
0:07:26 of information. Whereas with the SARS crisis, journalist Helen Branswell at Stat News was
0:07:31 commenting because she had covered the SARS crisis in 2003, that one might be tempted
0:07:35 to say SARS start was worse, it spread faster, not sure that’s true. SARS was well underway
0:07:40 for at least 4.5 months before the world knew there was a new virus spreading. This current
0:07:44 coronavirus seems to have been spotted much, much sooner after its emergence. But what’s
0:07:47 really interesting is not just that it’s been spotted sooner, but that the genetic
0:07:53 information we have is moving much faster. So can you talk to me about what that tells
0:07:54 us and why that matters?
0:07:58 So there’s a couple of areas where you get benefits from having all this genetic information
0:08:03 so quickly. The first is just diagnosis. So if tomorrow somebody in San Francisco was
0:08:07 to go into an urgent care clinic and say that they have a cold, we could really quickly
0:08:12 identify whether or not that actually belongs to this epidemic.
0:08:16 Another advantage we have once we have the genome sequence is that in this age of genomic
0:08:20 medicine that we’re entering, where we’re actually creating vaccines that are based
0:08:26 on genome sequences. The third implication is for figuring out treatments and also predicting
0:08:30 some of the features of the epidemic. So we know in this example that this coronavirus
0:08:35 looks really similar to SARS. So we can look back at the SARS epidemic, understand how
0:08:39 quickly it’s spread, in which populations. And from the genomic information, you can
0:08:44 actually see, for example, the spikes on the corona, which are involved in getting into
0:08:49 cells, do those look similar to what the SARS spikes look like? And maybe the treatments
0:08:51 that work in this case as well.
0:08:54 So the high level summary is that having the genomic information, which we didn’t have
0:08:59 then when we do have now, by then I mean the SARS outbreak, which happened about 2002 to
0:09:05 2004, the peak was 2003, is that you can classify things much more easily, figure out where
0:09:10 it belongs, it doesn’t belong, kind of isolate that, that you can develop things faster based
0:09:14 on it, although there isn’t a vaccine yet, and that you can figure out treatment protocols
0:09:17 based on the similarities and differences.
0:09:21 Was there anything else on the connection between mayors and SARS from a genomics perspective?
0:09:26 Yeah. So because the science is happening live, you’re seeing a lot of pretty quick modifications
0:09:31 to what people have already said. So the first paper analyzing the genome of this virus that
0:09:38 I saw at least, described that the spike proteins that are used to enter into lung cells are
0:09:42 different enough between SARS and this new coronavirus, that they thought it wouldn’t
0:09:47 be as bad as SARS. And then within like literally two days, I think I saw a paper that corrected
0:09:51 that and said that actually the protein is quite similar at the protein level.
0:09:54 Are there any bioengineering implications of that? This goes to me that the eternal
0:09:58 question of how DNA expresses itself practically in the complexity of disease.
0:10:02 That’s a really good question. This mirrors everything in genomics where we thought that
0:10:05 once we have the sequence, we’ll know all the answers. And that’s definitely not the
0:10:09 case. We can know from the DNA what the difference is going to be in the protein. That’s one
0:10:13 to one, there’s no guessing there. But once we know what’s different about the protein,
0:10:18 we don’t yet know quite what that means for how it will behave. So we don’t know if that
0:10:23 difference means it’s stronger or it’s weaker or if it will infect different cells or what
0:10:24 that’s going to mean.
0:10:28 So now let’s move on to more of the details of how it spreads and the measurements of
0:10:33 that spread. So first note is that generally the coronavirus has spread through air. They’re
0:10:39 known as zoonotic in that they originate in animals and only sometimes leap to humans.
0:10:43 And there’s been some speculation that this one seemed to originate in bats, but it’s
0:10:48 usually indirect mechanisms. So in mayors, it went from bats to camels before going to
0:10:52 humans. And a couple of papers have commented on the similarity of this new virus to bat
0:10:58 DNA. Like one found that it’s 96% identical. Another journal of medical virology also
0:11:02 observed similar components, but suggested snakes and many other people are skeptical
0:11:06 of that. The long story short is no one really knows, despite having some genomic information
0:11:07 around it.
0:11:11 But it’s interesting to note that important epidemics come from animal sources and some
0:11:15 speculation about the reason for that is that the viruses have time to evolve in their animal
0:11:19 hosts and they’re evolving away from what human hosts have seen before and what their
0:11:21 immune system has been able to recognize before.
0:11:27 So then let’s talk about the spread. A lot of the articles are talking about R0 or R0.
0:11:28 What does it measure?
0:11:33 So R0 is essentially the number of people that you would expect to get infected from
0:11:34 any single case of infection.
0:11:37 So if the R0 is say five, what does that mean?
0:11:41 That means that you should expect that on average, five people will get sick from one
0:11:45 single person that they come into contact with. So for every person that has the disease,
0:11:47 five additional people get the disease.
0:11:52 And interestingly, these are reported as ranges. So like measles has an R0 of 12 to 18.
0:11:57 Yeah. So measles is super infectious. It’s known as sort of the highest or one of the
0:12:01 highest infectiousness variables, which is why it was so important to have vaccines and
0:12:03 herd immunity for measles specifically.
0:12:08 And the reason that it’s reported as ranges is because they depend on the particular population
0:12:10 and the particular moment in time.
0:12:15 So then let’s just talk about some of the facts of the spread. So the 29 exported cases reported
0:12:21 by the World Health Organization, 26 had a travel history from Wuhan city in China.
0:12:24 And then for two of the three cases that were identified in countries outside of China,
0:12:29 one in Australia had direct contact with the confirmed case from Wuhan while in China.
0:12:33 And one in Vietnam had no travel history, but was in contact with the confirmed case.
0:12:35 His father had a travel history to Wuhan.
0:12:39 So what we do know is that human to human transmission is occurring. The preliminary
0:12:42 are not estimate that was presented at the International Health Regulations Emergency
0:12:49 Committee was a range of 1.4 to 2.5, which relatively to the measles example is not as
0:12:54 crazy bad. SARS had an R not between two to five. So that kind of puts that those numbers
0:12:55 in perspective.
0:12:59 It might be a little premature to set these numbers just because the number of cases is
0:13:04 still been relatively low and they haven’t had time to play themselves out. So we don’t
0:13:08 know if there’s many, many cases out there who have not presented symptoms and therefore
0:13:12 we don’t have clear stats on those people.
0:13:17 The big question here is how bad is this epidemic? That’s the question with every epidemic. And
0:13:20 it’s actually so much more complicated than that because there’s a number of different
0:13:24 variables that go into determining how bad something is going to be. It’s very tempting
0:13:29 to put sort of a single number on how bad on a scale of one to 10, for example, but it
0:13:33 doesn’t really take into account all of the nuance in each particular epidemic.
0:13:38 So our not is calculated from the data is actually an aggregate measure. There’s a lot
0:13:41 of different variables that go into our not. So to break them all down into their individual
0:13:44 components and then we can build them back up into our not.
0:13:48 There’s a lot of variables that are going to matter here. One is how well does the virus
0:13:53 transmit itself? So if it’s airborne, it’s able to multiply or transmit itself substantially
0:13:54 more easily.
0:13:59 Right. Versus like exchanging bodily fluids and which requires a lot of specific contact.
0:14:03 Exactly. Another piece is how is it actually getting into the cells? Is it good at infecting
0:14:09 cells? Is it good at its job essentially? Another question is what is the population that it’s
0:14:15 occurring inside of? Is that population moving a lot? Are people coming into very close contact
0:14:20 with each other? So that’s also going to factor in particularly interesting feature of this
0:14:24 is that it happened during Chinese New Year, which is a period of time when a lot of people
0:14:26 in China travel.
0:14:29 So what I think is really interesting to this and having worked at the Gates Foundation and
0:14:35 seen how we think about epidemics on a global scale is that there’s different, there’s two
0:14:39 orthogonal ways to think about an epidemic, which is how much does it spread and then
0:14:41 how bad is it once you get it.
0:14:44 And you’re saying it’s orthogonal or contradictory or why?
0:14:49 So there’s a notion of case fatality, which is for each infection with what likelihood
0:14:53 will the person die from that infection or have like very serious complications. And that’s
0:14:57 actually completely orthogonal to all of the other variables that we talked about before.
0:15:03 One is that if the virus is actually not that deadly or if it expresses itself in a person
0:15:07 after a substantial incubation sign, it might end up creating a bigger epidemic because
0:15:13 that additional time will allow the patient to infect additional individuals. The are
0:15:19 not for that particular virus might be substantially higher, even though its fatality rate is lower.
0:15:23 So it’s an interesting paradox that you could actually have a virus that is less bad once
0:15:26 you get it, but is more bad on the population scale.
0:15:30 So you have to define the metric by which you’re saying whether or not an epidemic is
0:15:34 bad. Is it the number of people who die? Is it the number of people who are infected?
0:15:38 Is it the extent of the spread geographically? There’s a lot of different ways to think about
0:15:40 how bad an epidemic is.
0:15:44 Now let’s just talk about treatments and concrete things that are happening right now.
0:15:48 So just to quickly summarize what’s happening. The CDC is conducting entry skinnings at five
0:15:55 major airports Atlanta, Chicago, Los Angeles, LAX, New York City, JFK and San Francisco.
0:15:58 Doctors are treating symptoms. There’s no vaccine yet. The CDC has developed a real-time
0:16:06 reverse transcription polymerase chain reaction or RRT-PCR test that can diagnose this virus
0:16:08 in respiratory and serum samples.
0:16:15 So what that is is basically a way of seeing if the nucleic acids, if the patient sample
0:16:19 contains the same sequence as the sequence that we know to be involved with the virus
0:16:23 that we know as a part of the virus. So you don’t have to sequence every single patient.
0:16:28 Okay, and then on January 24th, just a few days ago, the CDC publicly posted the assay
0:16:32 protocol for this test and the quote they said, “Currently testing for this virus must
0:16:36 take place at the CDC, but in the coming days and weeks, they will share these tests with
0:16:40 domestic and international partners. And they’re also growing the virus and cell culture, which
0:16:44 is necessary for further studies, including for additional characterization.”
0:16:49 So concretely now from a practical technology point of view, because obviously this is the
0:16:53 serious crisis. There’s a lot to be done and a lot of different players. Where do you sort
0:16:56 of seeing some of the things that might happen that where tech can help?
0:17:01 What’s really interesting about this moment is that because we have this increase in genomic
0:17:08 medicine, just this past weekend, the Coalition for Epidemic Preparedness Innovations, CEPI,
0:17:13 gave out grants to three different pharmaceutical companies of a total of $12.5 million, and
0:17:17 they’re currently engaged in a race. These companies are targeting dates for releasing
0:17:22 their vaccine of between four to 16 weeks from now, which is just totally unheard of.
0:17:27 For a company to spin up a vaccine for the SARS epidemic in 2003 would have taken months
0:17:32 to years for them to develop the new drug and actually get it approved. In this case,
0:17:36 two of these companies made these types of vaccines for other sequences. They’re able
0:17:40 to take what they’ve already built in-house for their other programs, and they can just
0:17:44 very quickly adapt it to this particular virus. All they have to do is create a drop-in replacement
0:17:49 of the sequence that they’ve already worked on with the sequence of this new coronavirus.
0:17:55 Is it fair to say that it’s not dissimilar to engineering in terms of semiconductor and
0:17:58 manufacturing lines? When you say drop-in sequence, does that mean that it’s basically
0:18:03 a matter of using existing scaling methods and you’re just changing the actual code,
0:18:04 quite literally the biological code?
0:18:09 I think that’s actually a really good way of describing it. It’s changing the code,
0:18:12 but you already have the manufacturing line set up. You already know exactly how you’re
0:18:17 going to make this thing. There may be differences, but they will be minimal compared to the differences
0:18:20 that would have existed with a completely different medicine.
0:18:22 Having to bespoke or custom-make it yourself.
0:18:23 Yeah, exactly.
0:18:28 Bottom-line it for me, Judy. How should we think about this news about the coronavirus
0:18:35 still developing? Just to be very clear. Specifically, this is for NCOV 2019. We’ve
0:18:39 covered the high level of what we know and what we don’t know. What would your bottom-line
0:18:42 be from the perspective of a bioengineer?
0:18:46 The bottom-line is that we really need to think about how we are interacting with people,
0:18:51 how we’re traveling, and how we are protecting ourselves from the virus. From my perspective
0:18:57 as a bioengineer, we’ve been talking about how sequencing and synthesis of DNA is becoming
0:19:02 faster and cheaper every single day. This is an example of that in action. This is not
0:19:06 something that would have been possible even a couple of years ago. I think that what we’re
0:19:10 seeing is the beginning of how quickly and how efficiently we’re going to be able to
0:19:16 get to vaccines in the future as we continue to decrease the cost of sequencing and synthesis.
0:19:20 This is a really interesting time because we’re able to figure out the diagnostics piece,
0:19:25 the vaccine piece, and the treatment piece. It’s still in progress, but we have a huge
0:19:26 head start.
0:19:33 Thank you, Judy. For those of you who would like more information, please visit www.cdc.gov/coronavirus.
0:19:38 I’ve also included the link sources for this episode in the show notes, which you can find
0:19:44 at a6nc.com/16minutes. As a reminder, if you haven’t already subscribed to this separate
0:19:48 show in your podcast feed, please do so now and thank you for listening.
This episode of 16 Minutes on the news from a16z is all about the recent coronavirus outbreak — or rather, a new type of coronavirus called 2019-nCoV for 2019 novel coronavirus. Since it’s an ongoing and fast-developing news cycle, we take a quick snapshot for where we are, what we know, and what we don’t know, and discuss the vantage point of where tech comes in. Topics covered include:
- definition of a virus, categories of coronaviruses
- origins and spread
- how this stacks up so far against SARS and MERS
- speed of sequencing, implications of genomic info
- speed of information sharing
- R0 (“r-naught”/”nought”) and what it measures
- different ways to think about how bad a given epidemic is
- current moves and treatments
Our a16z guest is Judy Savitskaya on the bio team, in conversation with Sonal Chokshi.
Link sources or background readings for this episode:
- Centers for Disease Control and Prevention (in the U.S. Department of Health and Human Services) + types
- World Health Organization (in the United Nations) — situation report #6, January 26
Other background readings / pieces mentioned in this episode:
- “Scientists are moving at record speed to create new coronavirus vaccines–but they may come too late”, Jon Cohen, Science (AAAS), January 27
- “Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China”, The Lancet, January 24
- “Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin”, bioRxiv, January 2 *note – preprint, NOT peer reviewed*
- “The deceptively simple number sparking coronavirus fears”, Ed Yong, The Atlantic, January 28 *this appeared AFTER this episode was recorded, so sharing here as additional reading only*
image: CDC

Leave a Reply
You must be logged in to post a comment.